C57BL/6 Mouse Primary Hepatocytes - Plateable
_______________________________________________________________________________________
C57BL/6 Mouse Primary Hepatocytes - Plateable
Catalog No. C57-6224F
Suggested Medium
M1365 Complete Hepatocyte Medium /w Kit (500 ml)
Product Description
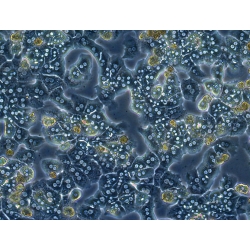
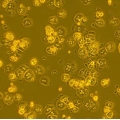
C57BL/6 Mouse Primary Hepatocytes from Cell Biologics are isolated from liver tissues of pathogen-free laboratory C57BL/6 mice. Cells at passage 0 are cryo-preserved and each vial contains 3x106 cells per ml. C57BL/6 Mouse Primary Hepatocytes are characterized by immunofluorescence staining with an antibody of ZO-1. Cells can be plated on culture plates for desired experiments under the cell culture conditions specified by Cell Biologics. Primary hepatocytes can not be cultured indefinitely and can not be passaged.
Storage
Cryopreserved Cells are shipped with dry ice overnight. Upon arrival, transfer frozen cells to liquid nitrogen (-150°C) immediately until ready for use. Cells should be plated and used directly for desired studies within 1-2 weeks upon receipt. Primary cells can never be kept at -20 °C or -80 °C freezer.
Authorized Uses of Cell Biologics’ Products
C57BL/6 Mouse Primary Hepatocytes from Cell Biologics are distributed for research purposes only. Our products are not authorized for human use, for in vitro diagnostic or therapeutic procedures. Transfer or resale of any Cell Biologics’ cells or products from the purchaser to other markets, organizations or individuals is prohibited by Cell Biologics without the company’s written consent. Cell Biologics’ Terms and Conditions must be accepted before submitting an order.
Disclaimer
Investigators should handle the cells with caution and treat all animal cells as potential pathogens, since no test procedure can completely guarantee the absence of infectious agents.
Warranty and Liability
Cell Biologics’ guarantee applies only to your purchase of Cell Biologics’ Cells with Cell Biologics’ Media and Coating Solution for appropriate cell culture and cell testing following Cell Biologics’ online protocols within 35 days from the date of product delivery.
_____________________________________________________________________________________
Primary Hepatocyte Culture Protocol
All cell culture procedures must be conducted in a bio-safety cabinet.
Any and all media, supplements, and reagents must be sterilized by filtration through a 0.2 µm filter.
Use aseptic technique to prevent microbial contamination.
Medium
Review the information provided on the Cell Biologics website about appropriate culture media (e.g. serum and other supplements). Use pre-warmed (37°C) cell culture media (30-50 ML, Catalog No. M1365) to seed cells and when changing media.
Coating of Cell Culture Plates or Dishes
Coat sterile culture dishes or flasks with Gelatin Coating Solution (Catalog No. 6950, Cell Biologics) for 2 min, then aspirate the excess solution.
Cell Recovery from Cryovial
- Thaw the cells quickly (<2 min) in a 37°C water bath until just prior to complete thawing.
- Wipe the outside of the vial with 70% ethanol.
- Gently resuspend the cells and transfer cells (3 million cells) to a 7 ml sterile tube.
- Add 7 ml of pre-warmed Cell Biologics’ Cell Culture Medium (Catalog number M1365, Cell Biologics) gently to the side of the tube and slowly pipette up and down 2 to 3 times to resuspend the cells.
- Carefully pour cell suspension into 100 mm dish (or 0.6-1.0 million cells per well of 6-well plate).
- Once the cells have attached to the culture dishes about 70-80% (after 2-8h), gently aspirate supernatant (remove cell debris and non-adherent cells) and replace the pre-warmed Maintenance Medium (Catalog number M1365, Cell Biologics).
- Cells should be checked daily under a microscope to verify appropriate cell morphology.
- Proceed with experiment assays within 1-3 days (when cells have reached 90-100% confluence) after plating cells.
Note: The numbers of seeding cells in each well may need to be modified according to the user's experience. Recommended seeding density: 200,000 viable cells/cm2.
Publication;
Front Pharmacol. 2018; 9: 1376.







 Categories
Categories Shopping Cart
Shopping Cart Information
Information