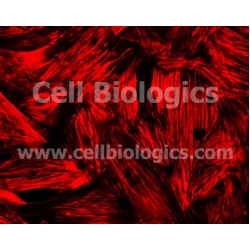
Porcine Primary Kidney Smooth Muscle Cells
Porcine Primary Kidney Smooth Muscle Cells
Catalog No. P-6116
Suggested Medium: Smooth Muscle Cell Medium /w Kit –500 ML
Catalog No. M2268
Product Description
Porcine Primary Kidney Smooth Muscle Cells from Cell Biologics are isolated from Porcine Kidney tissue. Porcine Primary Kidney Smooth Muscle Cells are grown in T25 tissue culture flasks pre-coated with gelatin-based coating solution for 2 min and incubated in Cell Biologics Culture Complete Growth Medium generally for 3-7 days. Prior to shipping, cells at passage 1 are detached from the culture flasks and immediately cryo-preserved in vials. Each vial contains at least 0.5x106 cells per ml and is delivered frozen. The cells are characterized by immunofluorescent method with antibodies to α-smooth muscle actin. Porcine Primary Kidney Smooth Muscle Cells are negative for bacteria, yeast, fungi, and mycoplasma. Cells can be expanded on a multiwell culture plate ready for experiments (or cells may be expanded for 2 passages at a split ratio of 1:2) under the cell culture conditions specified by Cell Biologics. Repeated freezing and thawing of cells is not recommended.
Laboratory Applications
Porcine Primary Kidney Smooth Muscle Cells can be used in assays of standard biochemical procedures performed with cell cultures include RT-PCR, Western blotting, immunoprecipitation, immunofluorescent flow cytometry, or generating cell derivatives for desired research applications.
Storage of Cell Biologics’ Products
Cell Biologics ships frozen cells on dry ice. On receipt, immediately transfer frozen cells to liquid nitrogen (-180 °C) until ready for experimental use. Live-cell shipment is also available on request.
Never can primary cells be kept at -20 °C.
Authorized Uses of Cell Biologics’ Products
Porcine Primary Kidney Smooth Muscle Cells from Cell Biologics are distributed for research purposes only. Our products are not authorized for human use, for in vitro diagnostic procedures, or for therapeutic procedures. Transfer or resale of any Cell Biologics’ cells or products from the purchaser to other markets, organizations or individuals is prohibited by Cell Biologics without the company’s written consent. Cell Biologics’ Terms and Conditions must be accepted before submitting an order.
Disclaimer
Appropriate safety procedures should always be used with this material. Although Porcine Primary Kidney Smooth Muscle Cells are isolated from normal human tissue, investigators should handle the cells that they receive from Cell Biologics with caution and treat all animal cells as potential pathogens, since no test procedure can completely guarantee the absence of infectious agents. The entire text of discussing Biosafety in Microbiological and Biomedical Laboratories, 5th ed. is available online at http://www.cdc.gov/biosafety/publications/bmbl5/index.htm.
Warranty and Liability
Cell Biologics’ guarantee applies only to your purchase of Cell Biologics’ cells with Cell Biologics’ Media and Coating Solution for appropriate cell culture and cell testing following Cell Biologics’ online protocols within 35 days from the date of product delivery.
Primary Cell Culture Protocol
All cell culture procedures must be conducted in a bio-safety cabinet.
Any and all media, supplements, and reagents must be sterilized by filtration through a 0.2 µm filter.
Use aseptic technique to prevent microbial contamination.
Cryo-preserved cells must be stored in liquid nitrogen or seeded immediately upon arrival.
Medium Review the information provided on the Cell Biologics’ website about appropriate culture media (e.g. serum and other supplements). Use pre-warmed (37°C) cell culture media (30-50 ML) to recover cryo-preserved cells and when changing media or splitting cells.
Coat sterile culture dishes or flasks with Gelatin-Based Coating Solution (Cell Biologics, Catalog No. 6950) for 2 min and then aspirate the excess solution before seeding cells.
When you receive the live cells in a T25 or T75 flask, remove the sticker from the filter cap, and keep the flask with 6-20 ml existing medium in 37°C CO2 incubator for 1 hour before replacing the desired Cell Biologics' cell culture medium. Either split the 95-100% confluent cells from a T25 flask to a T75 flask after 1 hour or let the cells grow in the T25 flask with the desired Medium (such as M2268) for 12-48 hours before subculturing cells. The recommended split ratio for primary cells is 1:2.
• Quickly thaw cells in cryo-vial by incubating them in a 37°C water bath for <1 min until there is just a small bit of ice left in the vial.
• Promptly remove the vial and wipe it down with 70% ethanol.
• Transfer cells from the vial to a sterile centrifuge tube. Add 8-10 ml of pre-warmed Cell Biologics’ Cell Culture Medium.
• Flush the vial with an additional 0.5-1 ml of medium to ensure complete transfer of cells to the centrifuge tube.
• Centrifuge cells at 120 g for 5 minutes.
• Aspirate the supernatant and resuspend the cell pellet in 6 ml of Cell Biologics’ Cell Culture Growth
Medium.
• Add resuspended cells into a T25 flask pre-coated with Gelatin-Based Coating Solution (Cell Biologics, Catalog No. 6950).
• Place the T25 flask in a humidified, 5%-CO2 incubator at 37°C.
• Change culture media the following day to remove non-adherent cells and replenish nutrients.
• Change cell culture medium every day when cells are >70% confluent.
• Cells should be checked daily under a microscope to verify appropriate cell morphology.
• Remove and discard the cell culture media from the flask.
• Flush the adherent layer 1-2 times using a 5 ml sterile pipette with sterile PBS (1X) without calcium and magnesium to dislodge loosely attached cells and remove fraction.
• Remove and discard the wash solution from the flask.
• Incubate cells with warm (37°C) 0.05% Trypsin-EDTA solution (Cell Biologics, Catalog No. 6915) for 2-5 minutes. Use 3.0 ml of Trypsin-EDTA solution when collecting cells from a T75 flask, and 2 ml
when using a T25 flask. As soon as cells have detached (the flask may require a few firm gentle
taps), add 8-10 ml of Cell Biologics’ Cell Culture Medium supplemented with 5-10 % FBS to a T25 or T75 flask (the FBS will neutralize the trypsin).
• Plate cells in fresh flasks or plates precoated with Gelatin-Based Coating Solution in a humidified, 5%-CO2 incubator at 37°C.
• Change culture media the following day to remove non-adherent cells and replenish nutrients.
• Cells should be checked daily under a microscopy to verify appropriate cell morphology.
• Change culture medium every 24-48 hours. Please note that the medium should be changed every day
when cells are >70% confluent to remove non-adherent cells and replenish nutrients. Pre-wash cells
with 1X PBS 1-2 times whenever replacing the medium.
Procedure for Freezing Cells
Materials:
• 1X Phosphate Buffered Saline (PBS-1X)
• 0.05% Trypsin-EDTA (1X) solution (Cell Biologics, Catalog No. 6915)
• Tissue Culture Media
• Cold Freezing Media (10% DMSO, 50% FBS and 40% culture medium, Cell Biologics, Catalog No. 6916).
• Labeled Cryovials
• Confluent Cells
------------------------------------------------------------------
• Cells should be checked daily under a microscopy to verify appropriate cell morphology.
• Remove and discard the cell culture media from the flask.
• Flush the adherent layer with a 5 ml sterile pipette 1-2 times with sterile PBS (1X) without calcium and magnesium to dislodge loosely attached cells and remove fraction.
• Remove and discard the wash solution from the flask.
• Incubate cells with warm (37°C) 0.05% Trypsin-EDTA solution (Cell Biologics, Catalog No. 6915) for 2-5 minutes. Use 3.0 ml of 0.05% Trypsin-EDTA solution when collecting cells from a T75 flask, and 2 ml when using a T25 flask. As soon as cells have detached (the flask may require a few firm gentle taps), add 10 ml of Cell Culture Medium supplemented with 5-10 % FBS to the flask (the FBS will neutralize the trypsin).
• Centrifuge the cell suspension at 120 g for 5 minutes.
• Remove supernatant with sterile Pasteur pipette.
• Quickly re-suspend pellet by adding 1 ml freezing media per vial to be frozen.
• Place vials in Nalgene "Mr. Frosty" freezing container containing100% isopropyl alcohol at -70-80 °C for 24 h.
• Transfer vials to liquid N2 tank for indefinite storage.
We recommend freezing primary cells at the follow ratio
• A confluent primary cells grown in a T75 flask may be frozen in 3 cryovials.
• A confluent primary cells grown in a T25 flask may be frozen in 1-2 cryovials.
| Related Products | ||
| Cat. # | Name | |







 Categories
Categories Shopping Cart
Shopping Cart Information
Information