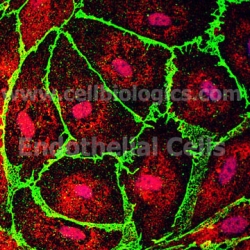
Primary Endothelial Cell Culture Protocol
Primary Endothelial Cell Culture Protocol
Primary Endothelial Cell Culture Protocol
● All cell culture procedures must be conducted in the bio-safety cabinet.
● All cell culture media, supplements, and reagents must be sterilized by filtration through a 0.2 µm filter.
● Use aseptic techniques to prevent microbial contamination.
● Upon receiving the package, cryopreserved cells should be immediately transferred from the dry ice shipping container to a liquid nitrogen storage tank.
Medium
● Review the information provided on the Cell Biologics website for appropriate complete culture media (e.g. serum and other supplements).
● Only pre-warm (37°C) the amount of cell culture media you will be using right away. 5 ML for a T25 or 8-10 ML for a T75 flask is needed when recovering cryo-preserved cells, changing media, or splitting cells.
● Repeated warming of complete culture media is not recommended.
Coating of Flasks or Dishes
Coat sterile culture dishes or flasks with Gelatin-Based Coating Solution (Cell Biologics, Catalog No. 6950) for 2 min and then aspirate the excess solution before seeding cells.
Handling of Live Cells upon arrival
● Upon receiving the live cells in a T25 or a T75 flask, remove the sticker from the filter cap, place the flask with 6-20 ml of the existing medium into 37°C, 5% CO2 incubator for 1 hour, then replace the medium with desired Cell Biologics' pre-warm (37°C) cell culture medium.
● These cells at 90% confluence can be split at 1:2 ratio for subculturing or frozen down for future usage when 95-100% confluent.
Cell Recovery from Cryovial
-
Quickly thaw cells by placing the vial in a 37°C water bath for <1 min or until there is just a small bit of ice left in the vial.
-
Promptly remove the vial and wipe it down with 70% ethanol.
-
Transfer cells from the vial to a 15 ml sterile conical centrifuge tube with 5 ml of pre-warmed Cell Biologics Cell Culture Medium.
-
Flush the vial with an additional 0.5-1 ml of medium to collect additional cells left in the vial.
-
Centrifuge the cells at 200 x g for 5 minutes. Note: Centrifugation of cells after thawing to remove residual DMSO is recommended.
-
Carefully aspirate the supernatant and resuspend the cell pellet with 5 ml of Cell Biologics’ Cell Culture Growth Medium. Add resuspended cells into a T25 flask pre-coated with Gelatin-Based Coating Solution (Cell Biologics, Catalog No. 6950).
-
Place the seeded T25 flask in a humidified, 5% CO2 incubator at 37°C. Leave the culture undisturbed for 12-16 hours.
-
Change culture media the following day and then every 24 hours afterwards to remove non-adherent cells and replenish nutrients (5 ML for a T25 flask or 10 ML for a T75 flask is needed).
-
Cells should be checked daily under a microscope for cell confluence and appropriate cell morphology.
Expansion of Cultured Primary Cells
-
Remove culture medium and wash the cells at room temperature 1X phosphate buffered saline solution (without calcium and magnesium).
-
Incubate the cells with pre-warmed (37°C) 0.25% Trypsin-EDTA solution (Cell Biologics, Catalog No. 6914) for 3-5 minutes. Use 3.0 ml of Trypsin-EDTA solution for a T75 flask and 2 ml for a T25 flask, respectively. As soon as the cells have detached (a few firm gentle taps on side of flask may help detachment), add 5-10 ml of Cell Biologics’ Cell Culture Medium to the flask (the FBS will neutralize the trypsin) and gently pipette up and down a few times.
-
Seed a vial of frozen cells into a T25 flasks/or a 60mm Cell Culture Dish (TC-treated polystyrene) precoated with Gelatin, and then return flasks/dishes into a humidified, 37°C, 5% CO2 incubator.
-
Change culture media the following day and then every 24 hours afterwards to remove non-adherent cells and replenish nutrients (The medium of 5 ML for a T25 or 10 ML for a T75 flask is needed).
-
Cells should be checked daily under a microscope for cell confluence and appropriate cell morphology.
-
Cells can be pre-washed 1-2 times with 1X PBS whenever replacing the medium (optional).
To seed a vial of frozen cells into a T25 flask, you can follow these steps:
· Thaw the cells: Put the frozen vial in a 37°C water bath until the cells are almost completely thawed, which can take around one minute.
· Resuspend the cells: Transfer the cells from the vial to a 15 mL conical tube that contains pre-warmed cell culture medium.
· Pellet the cells: Centrifuge the cells for 3–5 minutes at 1,500 rpm to pellet them.
· Transfer the cells to the flask: Discard the supernatant and resuspend the cells in the remaining cell culture medium. Then, transfer the cells to a T25 flask that contains pre-warmed cell culture medium.
· Pipette to mix: Use a pipette to mix the solution in the flask so the cells can be easily seeded.
· Place in the incubator: Put the flask in an incubator that's set to 37°C and 5% CO2.
We recommend splitting primary cells at the following ratio
• The recommended split ratio for primary cells is 1:2.
Procedure for Freezing Cells
Materials:
-
1X Phosphate Buffered Saline (PBS-1X)
-
0.25% Trypsin-EDTA (1X) solution (Cell Biologics, Catalog No. 6914)
-
Tissue Culture Media
-
Cold Freezing Media (10% DMSO, 50% FBS and 40% culture medium, Cell Biologics, Catalog No. 6916).
-
Labeled Cryovials
-
Confluent Cells
------------------------------------------------------------------
-
Remove and discard the cell culture media from the flask.
-
Flush the adherent layer 2 times with 7 ml sterile PBS (1X, without calcium and magnesium) to remove residual medium.
-
Incubate the cells with pre-warmed (37°C) 0.25% Trypsin-EDTA solution (Cell Biologics, Catalog No. 6914) for 3-5 minutes. Use 3.0 ml of Trypsin-EDTA solution for a T75 flask and 2 ml for a T25 flask, respectively. As soon as the cells have detached (a few firm gentle taps on side of flask may help detachment), add 5 ml of Cell Biologics’ Cell Culture Medium to the flask (the FBS will neutralize the trypsin) and gently pipette up and down 1-2 times.
-
Transfer the cell suspension to a centrifuge tube.
-
Centrifuge cells at 200 x g for 5 minutes.
-
Carefully remove supernatant with sterile Pasteur pipette.
-
Quickly resuspend the cell pellet by adding 1 ml freezing media per vial to be frozen.
-
Place vials in Nalgene "Mr. Frosty" freezing container containing100% isopropyl alcohol at -80 °C for 6-12h.
-
Transfer vials to liquid nitrogen tank for indefinite storage.
We recommend freezing primary cells at the following ratio
• A confluent primary endothelial cell grown in a T75 flask may be frozen in 2-3 cryovials.
• A confluent primary endothelial cell grown in a T25 flask may be frozen in 1 cryovial.
• Please send us light microscope cell images (Cell confluence >70-80%) if you have any questions with cultured cells.
| Related Products | ||
| Cat. # | Name | |







 Categories
Categories Shopping Cart
Shopping Cart Information
Information